Status: Planning for Phase 2 trials in frontline and relapsed/refractory patients
Highlights
- Phase 2 trial ready after successful completion of a Phase 1b multi-center, multi-cohort dose expansion trial that evaluated safety, tolerability and efficacy in combination therapy and monotherapy for patients with Acute Myeloid Leukemia (AML)
- The Phase 2 program will evaluate frontline and relapsed/refractory AML patients, who will receive APVO436 in combination with venetoclax and azacitidine in patients who are venetoclax treatment naïve
Overexpression of CD123 is a mark of many forms of leukemia. Aptevo’s lead proprietary drug candidate, APVO436 is a bispecific CD3xCD123 ADAPTIR molecule that is designed to redirect the patients’ immune system to destroy leukemia cells expressing the surface target antigen CD123. This antibody-like recombinant protein therapeutic is designed to simultaneously engage both leukemia cells and T cells of the immune system to trigger the destruction of leukemia cells. APVO436 has been designed with an unique CD3 binding domain to reduce the likelihood and severity of cytokine release syndrome (CRS). APVO436 has received orphan drug designation for AML according to the Orphan Drug Act.
The Molecule
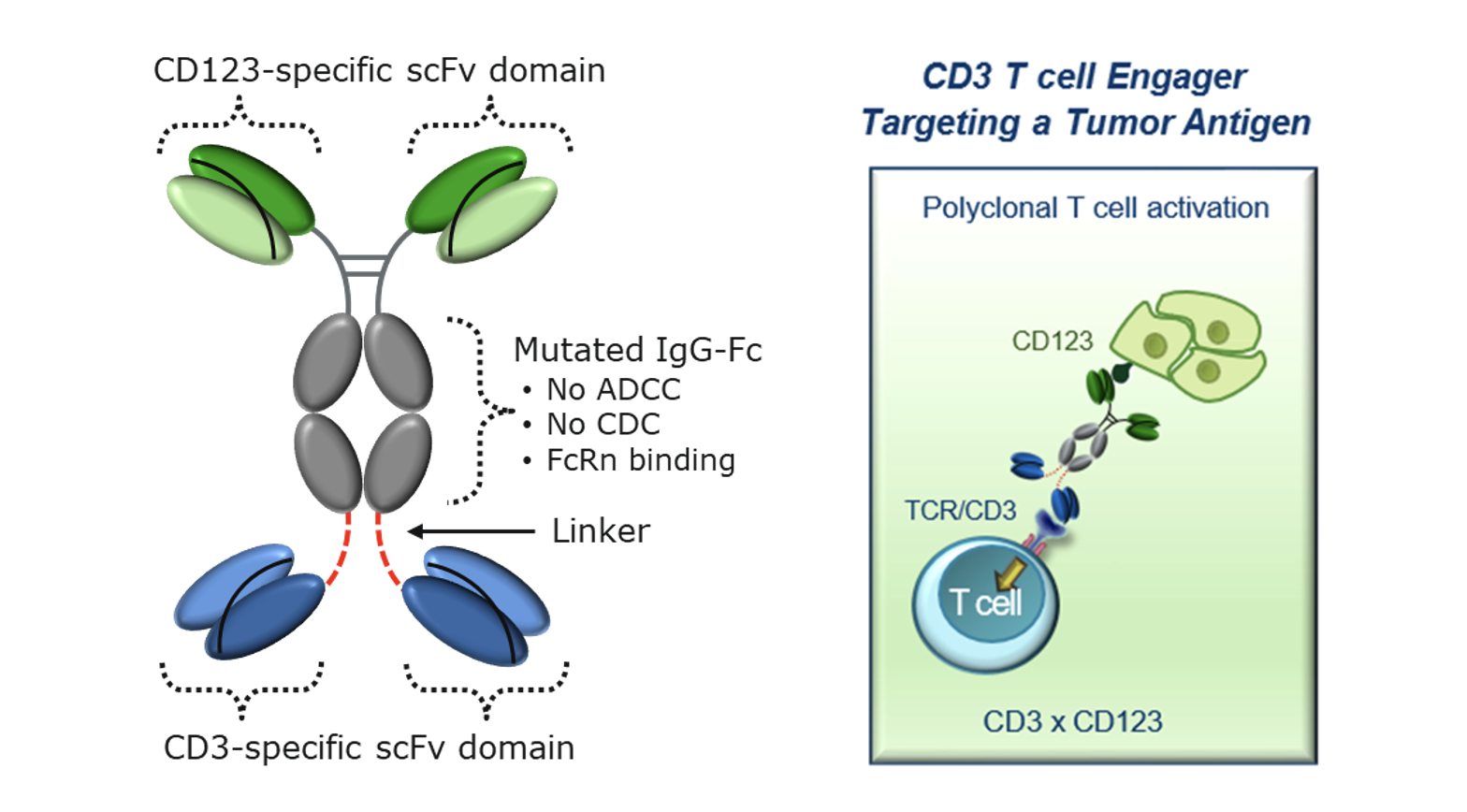
Potential advantages of APVO436 include:
Broad Utility: The tumor-associated antigen CD123 is expressed on several hematological malignancies including acute myeloid leukemia, acute lymphoblastic leukemia, hairy cell leukemia, myelodysplastic syndrome, and blastic plasmacytoid dendritic cell neoplasm, which all have significant unmet needs for safe and effective new therapies.
Reduced Toxicity: The unique CD3 binding domain has high potency tumor killing while inducing a reduced cytokine release profile in vitro. This approach has been shown clinically to provide a safer and more tolerable treatment for AML patients.
Clinical Trials
Phase 1b Expansion Trial: Design and Outcomes
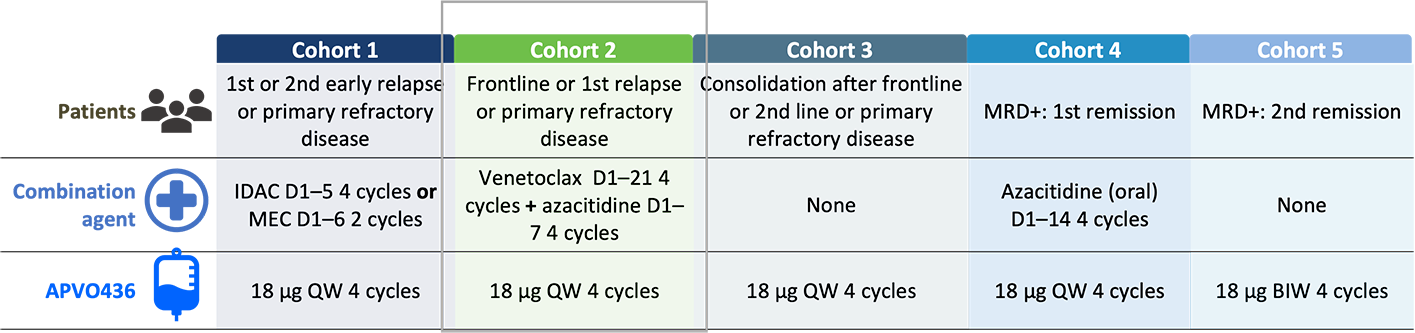
Trial Design Overview:
Evaluated patients (aged ≥ 18 years) with AML at different disease stages
STUDY ENDPOINTS:
Primary: Safety by evaluating Grade 3-4 TEAEs, SAEs, TEAEs of interest (CRS, IRRs, cardiac TEAEs and neurotoxicity)
Secondary: Efficacy by evaluating incidence of composite CR (CR + CRi + MLFS)
BIW, twice weekly; CR, complete remission; CRi; CR with incomplete hematologic recovery; CRS, cytokine release syndrome; D, day; IDAC, intermediate dose cytarabine; IRR, infusion-related reaction; MEC, mitoxantrone, etoposide, cytarabine; MLFS, morphologic leukemic-free state; MRD, minimal residual disease; QW, weekly; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Summary Of Clinical Activity:
- 91% clinical benefit in cohort 2 (combination of venetoclax + azacitidine + APVO436) in venetoclax treatment naïve patients
- Clinical benefit was approximately double the composite benchmark* across all clinical benefit categories**
- Monotherapy activity observed in both cohort 3 (monotherapy) and in the dose escalation trial
- *Aldoss 2019(PDF), Maiti 2021, Morsia 2020, Garciaz 2022, Feld 2021
- **Percentage of patients who achieved CR, CRi, MLFS and SD
Summary of Safety and Tolerability Outcomes:
- APVO436 Safety: Combination therapy that includes APVO436 + Venetoclax + Azacitidine (standard of care) is safe and well tolerated
- CRS was observed in fewer than one quarter of patients within the safety population and in most cases was mild or moderate (grade 1 or 2) and was manageable in the clinic
- Side effects were generally manageable and resolved while patients remained on treatment
- Results reinforce safety findings from the dose escalation trial and preclinical studies
View results from our presentation at ASH 2022
https://aptevotherapeutics.gcs-web.com/static-files/22408bdb-2e6f-4e84-b905-886aeb6ed277
Phase 1b Dose Escalation Trial
Prior to initiating the dose expansion trial, the Company conducted a Phase 1b dose escalation trial. The dose escalation trial included 46 patients with AML or myelodysplastic syndrome (MDS). The primary endpoint, identification of a recommended Phase 2 dose, was achieved. Additionally, APVO436 demonstrated manageable side effects and was well tolerated in the patient population. Signs of clinical activity – both stable disease and complete remissions, were also observed.
View results from our presentation at ASH 2021
https://aptevotherapeutics.gcs-web.com/static-files/638ab153-b1ff-49ee-b4b3-b1bed667bf73
View All APVO436 Scientific Presentations Here:
https://aptevotherapeutics.gcs-web.com/presentations


