Technology Overview
ADAPTIR™ and ADAPTIR-FLEX™ are proprietary platform technologies that produce novel drug candidates with the potential to improve outcomes for cancer patients.

ADAPTIR™ and ADAPTIR-FLEX™ are proprietary platform technologies that produce novel drug candidates with the potential to improve outcomes for cancer patients.
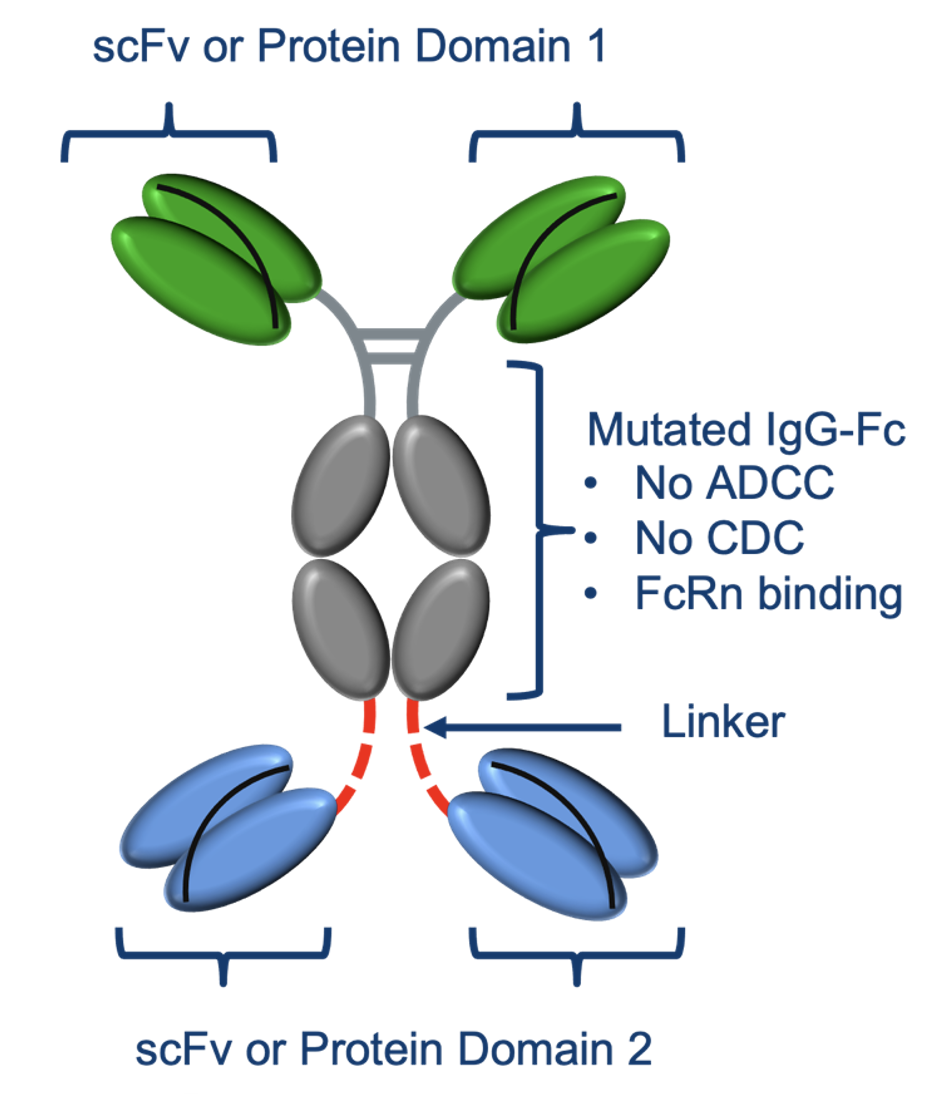
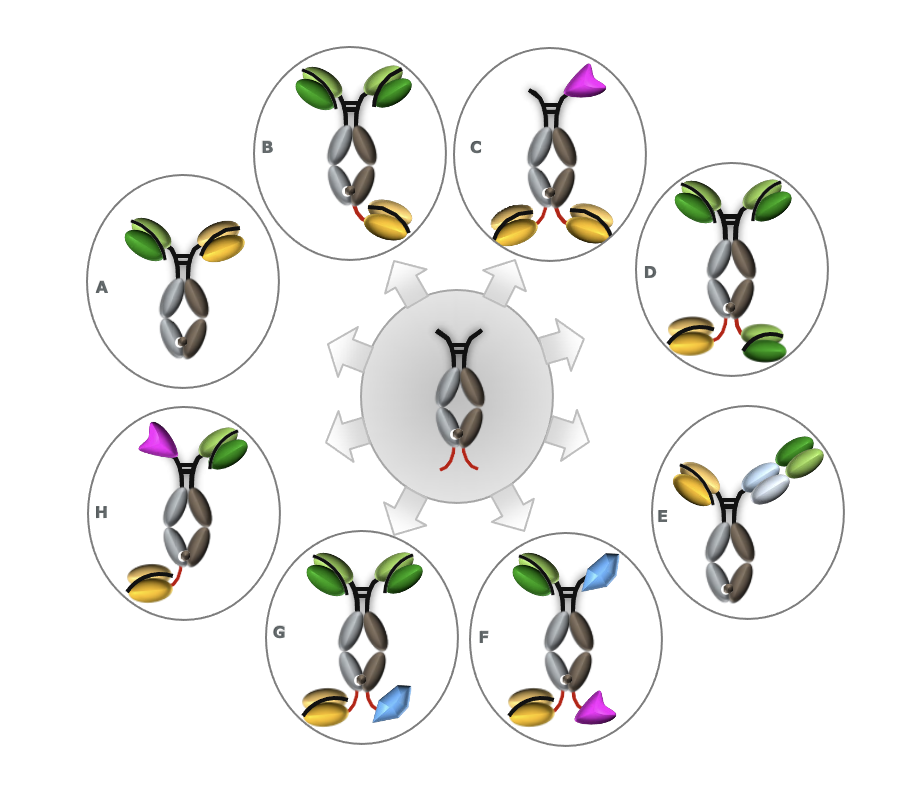
ADAPTIR Bispecifics are based on a robust technology platform with potential advantages over other platforms including; Increased stability and half-life, antibody-like manufacturing, ease of transfer and manufacturing at CMOs.
ADAPTIR T-cell engagers are based on a unique and proprietary anti-CD3.
Demonstrated potent tumor lysis in vitro and in vivo (preclinical models) and reduced cytokine release in preclinical studies may improve tolerability in a clinical setting
ADAPTIR and ADAPTIR-FLEX can be used to design therapeutics with multiple MOAs.
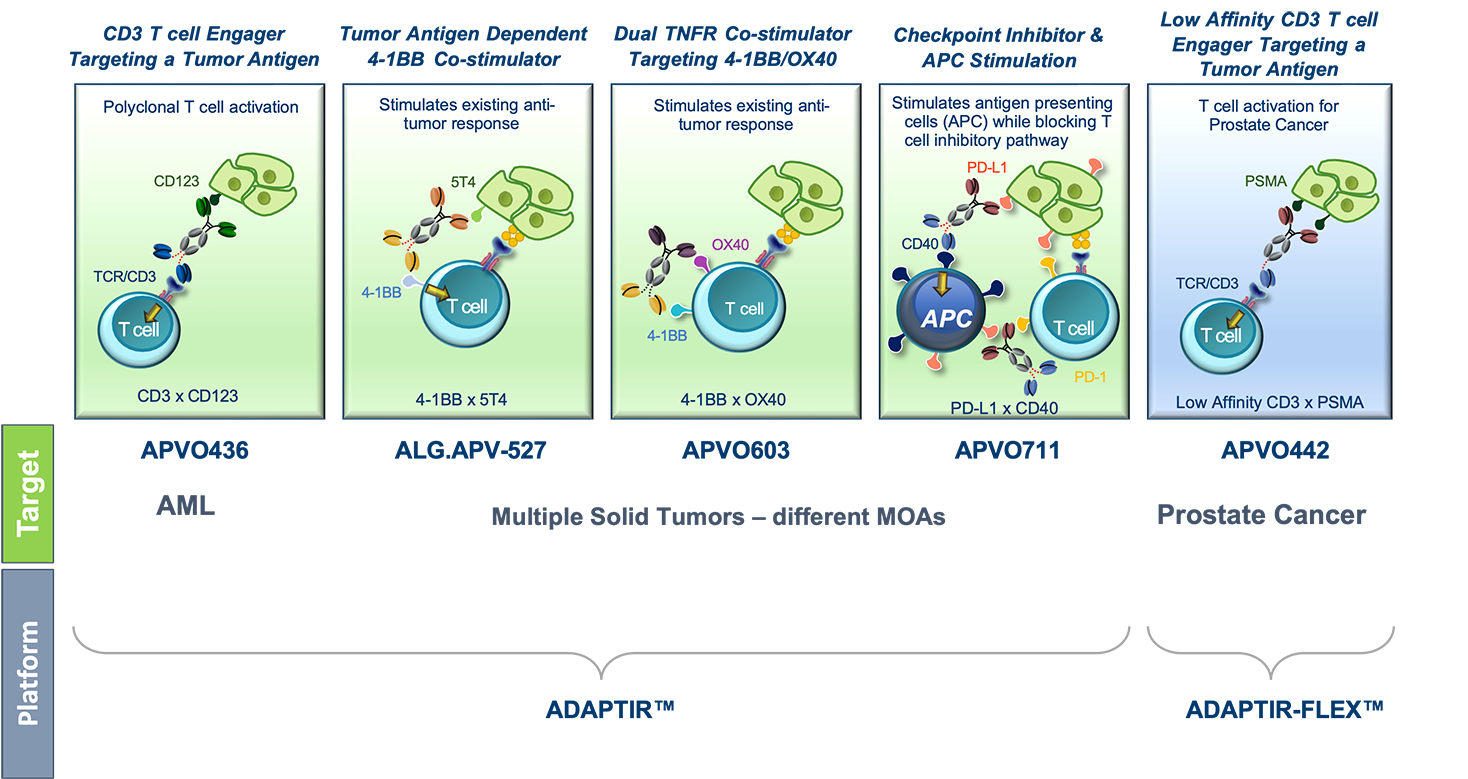
First, through generation of multiple anti-CD3 based T cell engagers for hematologic and solid tumors.Second, through utilization of TNFR superfamily members or other activating receptors (e.g.) Targeting cytokines to modulate the immune system for blocking immune checkpoint inhibitory signals to boost the immune response.
Modular and Flexible
Designed For Multiple Mechanisms of Action
Excellent Manufacturability Characteristics
Antibody-Like Half-Life
Multiple protein domains can be used in design of multi-specific candidates (single chain scFv, extracellular domain ECD, cytokines)