ALG.APV-527: 4-1BB x 5T4
Status: : Enrollment complete in Multi-Center, Multi-Cohort Phase 1 Trial for the treatment of solid tumors expressing the tumor-associated antigen 5T4
ABOUT THE MOLECULE
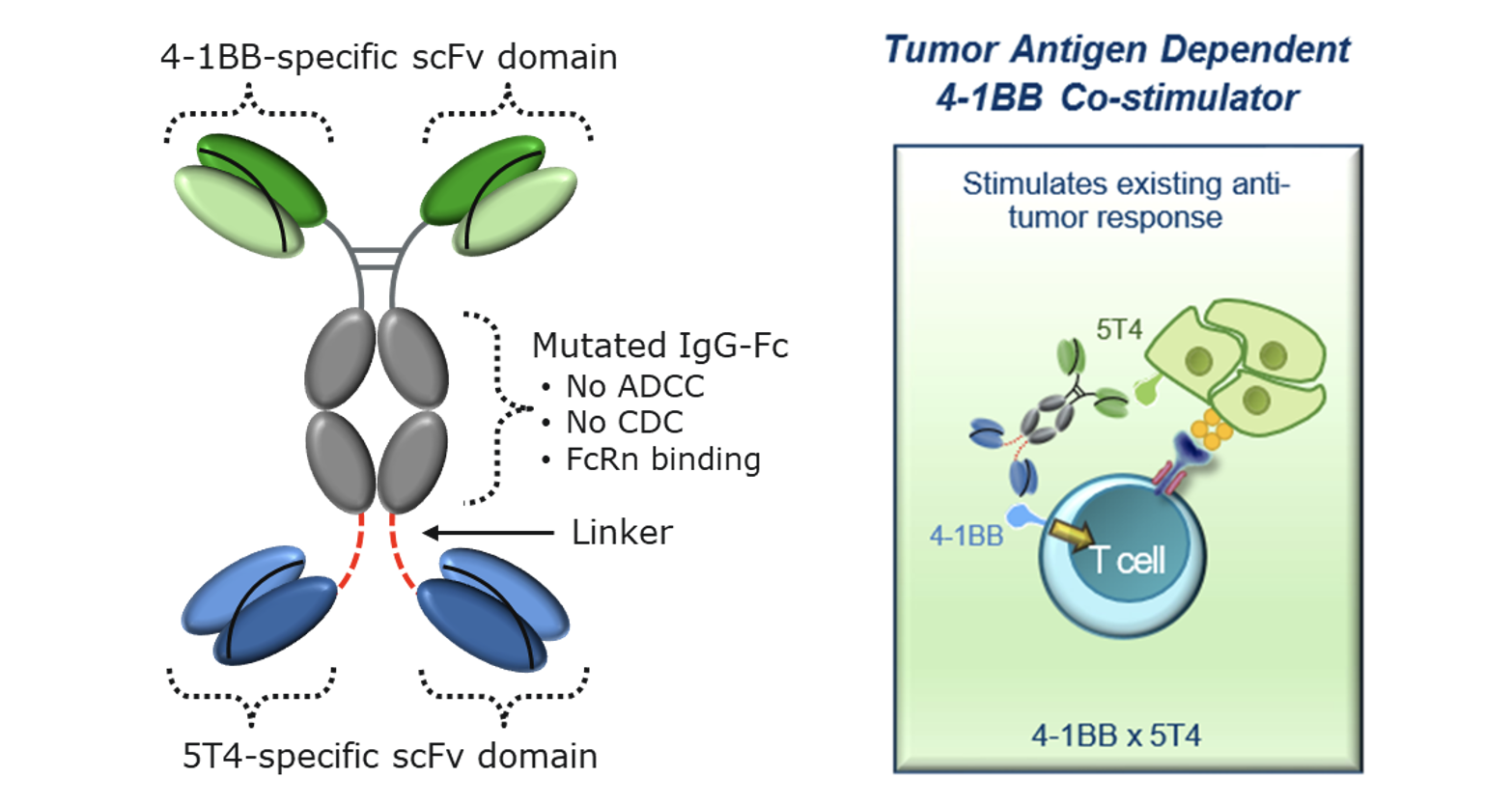
- APV-527 is a bispecific antibody that targets the immune co-stimulatory receptor 4-1BB and the tumor-associated antigen 5T4.
- The molecule is designed to target 5T4-expressing cancer cells by stimulating 4-1BB on both activated T cells and natural killer cells.
- APV-527 was designed to overcome limitations of the 1st generation 4-1BB monospecific mAbs:
- Clinical Activity: 5T4-dependent, 4-1BB signaling confers T cell and NK cell stimulation at the tumor site
- Safety: Conditional 4-1BB agonism is only active upon simultaneous binding to 4-1BB and 5T4, preventing peripheral immune cell activation in non-tumor tissues, which may improve safety profile in contrast to potential unspecific activation of 4-1BB with monospecific mABs, which trigger activation without a conditional second target activation.
PHASE 1 TRIAL DESIGN
The fully enrolled Phase 1 trial was conducted as a multi-center, multi-cohort, open-label dose-escalation trial that included administration of ALG.APV-527 in six escalating dose levels in a 3+3 design*. The trial enrolled adult patients with multiple solid tumor types/histologies likely to express the 5T4 antigen. ALG.APV-527 was administered intravenously once every two weeks and assessed the safety and tolerability, pharmacokinetics, pharmacodynamics and preliminary anti-tumor activity of ALG.APV-527.
*The 3+3 design proceeds in cohorts of three patients treated at increasing dose levels. Dose escalation stops when at least two out of three or six patients experience dose limiting toxicities (DLTs) at that dose level.
Clinical Highlights
Safety and Tolerability
- APV-527 demonstrated positive safety and tolerability across all cohorts
- No serious liver toxicity, a common side effect of other 4-1BB targeting treatments that can cause patients to discontinue dosing, was observed
- The drug was well tolerated across all dose levels
Clinical Activity/Efficacy
- Nine of 16 efficacy evaluable patients (56%) achieved stable disease (SD)
- One colon cancer patient achieved SD for more than six months
- The longest SD duration was in a breast cancer patient who entered the study with progressive disease, achieved stable disease and remained on study for >11 months. This patient successfully transitioned to a higher dose level twice
Evidence of biological activity of ALG.APV-527
- APV-527 could be measured in all patients. Serum concentrations of ALG.APV-527 were proportional to the administered dose
- Analysis of biomarkers in the serum of treated patients including soluble 4-1BB (surface protein found on certain immune cells) confirm biological activity of ALG.APV-527
- Analysis of biomarkers in biopsies (including the 5T4 target cells and CD8 T cancer killer cells are consistent with immune activation in the tumor microenvironment). This observation consistent with ALG.APV-527 expected MOA.
The Molecule
Collaboration: Co-developed by Aptevo Therapeutics and Alligator Biosciences under a collaboration agreement signed in 2017. ALG.APV-527 was built using Aptevo’s ADAPTIR bispecific technology platform and combines binding domains sourced from Alligator Biosciences’ ALLIGATOR-GOLD® human scFv library.
Broad Utility: 5T4 is a well-defined tumor antigen expressed on many different types of malignancies including, non-small cell lung, renal, pancreas, prostate, breast, colorectal, gastric, ovarian and cervical cancers. Conversely, 5T4 has limited expression on normal tissues, making it an attractive target for cancer immunotherapy.
Reduced Toxicity: ALG.APV-527 employs a novel mechanism of action to direct the therapeutic 4-1BB immune response towards the tumor, thereby potentially reducing the harmful side effects of systemic immune stimulation while providing a strong tumor-directed immune activation. ALG.APV-527 contains an Fc that does not interact with Fc gamma receptors, it has the potential to be less toxic than 1st generation monospecific 4-1BB antibodies.
Preclinical Studies
Preclinical data show that ALG.APV-527 has the potential to selectively activate and enhance tumor specific T cell responses at the tumor site without triggering systemic immune activation. Notably, these data show that ALG.APV-527:
- Stimulates increased T cell activation in the presence of 5T4-expressing cells
- Localizes to the site of 5T4 positive tumors in an in vivo melanoma tumor model
- Augments CD8 T cell proliferation and activation in a 5T4-dependent fashion
- Induces NK cell proliferation and enhances the NK cell cytotoxic profile, but only in the presence of 5T4
- Inhibits tumor growth in a human xenograft colon carcinoma model expressing the tumor antigen 5T4
- Was well tolerated in a dose-range finding pilot toxicology study with no major changes in liver enzyme levels, cytokine levels or immune cell populations
- Had an extended serum half-life of 5-7 days when administered by intravenous infusion in a preclinical toxicology study
View ALG-APV-527 Scientific Presentations Here:
https://aptevotherapeutics.gcs-web.com/presentations


