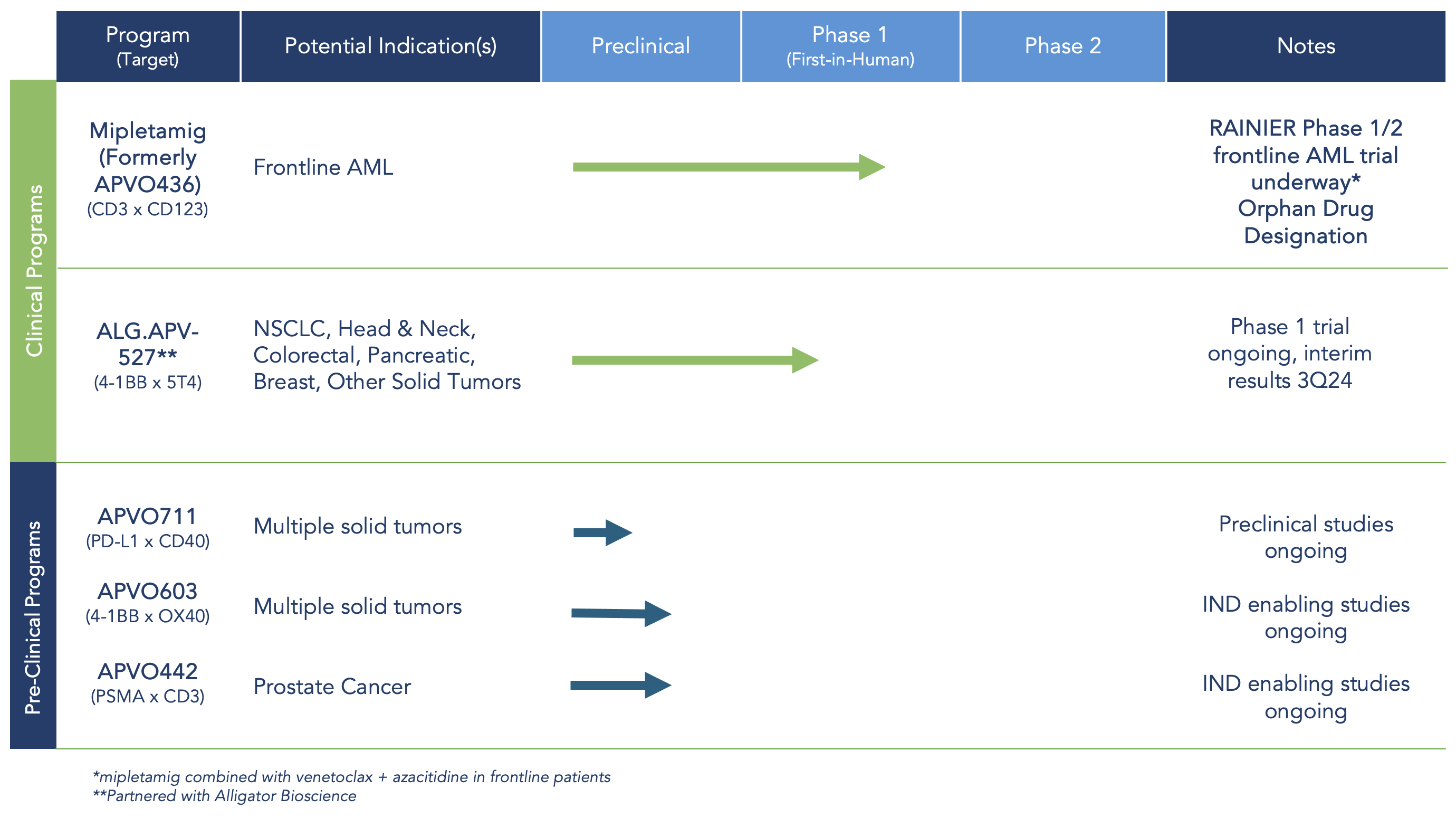
Therapeutic Candidate Portfolio: Proprietary and Powerful Drug Candidates for the Treatment of Blood and Solid Tumor Malignancies
Aptevo Therapeutics is committed to building a robust pipeline of preclinical ADAPTIR™ and ADAPTIR-FLEX™ bispecifics with a focus on novel mechanisms of action. By leveraging our proprietary platforms, these new candidates utilize multiple mechanisms of action, including redirected T cell cytotoxicity (RTCC), T cell co-stimulators, and exploit biological pathways for the development of novel cancer and autoimmune therapies.