APVO711 (PD-L1 x CD40):
Status: Preclinical Studies
Highlights
- Dual mechanism of action (i.e., blocks the PD-1/PD-L1 pathway and activates antigen presenting cells)
- Potential ability to combine with other drugs / therapeutics (i.e., multiple modalities)
- Validated pathway for PD-1/PD-L1 inhibitors set precedence for development route of APVO711
- Multi-billion-dollar market opportunity based on commercial success story of PD-1/PD-L1 inhibitors
The Molecule
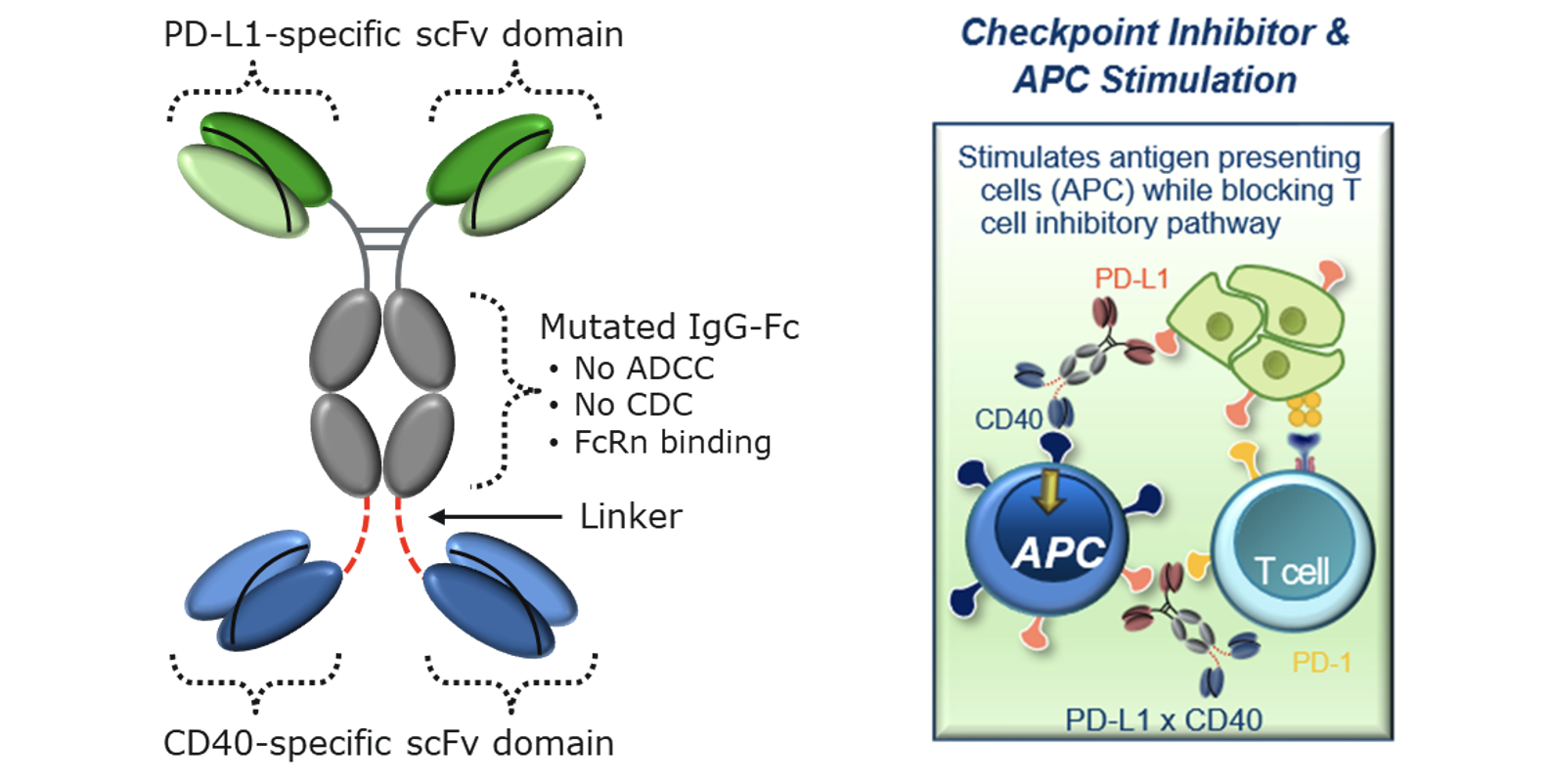
Potential advantages of APVO711 include:
- Broad Utility: APVO711 does not target a specific tumor antigen, thus allowing for broad potential therapeutic use for a number of different types of solid tumors
- Reduced Toxicity: Compared to first generation CD40 monoclonal antibodies that caused systemic toxicity, APVO711’s CD40 signaling only occurs when crosslinked through PD-L1
- Differentiation from either CD40 or PD-L1 Agonistic Monoclonal Antibodies: Because APVO711 contains an Fc that does not interact with Fc gamma receptors, it has the potential to be less toxic than monospecific CD40 antibodies, while still enhancing antigen presentation, cytokine secretion etc., which in turn enhances NK and T cell cytotoxicity. Beyond blocking the exhaustive effects of the PD-1/PD-L1 pathway on T cells, the PD-L1 binding domain can also be utilized as a tumor-targeting arm to enrich APVO711 into the tumor microenvironment
- Synergistic Activity: Designed to provide synergistic co-stimulation of CD40 on antigen presenting cells while simultaneously blocking PD-1/PD-L1 inhibitory pathway to potentially promote a more robust anti-tumor response


