Status: RAINIER dose optimization trial ongoing
About RAINIER
Aptevo’s RAINIER trial is a multi-center Phase 1b/2 study evaluating mipletamig, a first-in-class CD123 x CD3 bispecific antibody, in combination with venetoclax and azacitidine for newly diagnosed acute myeloid leukemia (AML) patients who are not candidates for intensive chemotherapy.
The study is designed to optimize dosing and confirm that mipletamig can be safely combined with the current standard of care while delivering meaningful clinical benefit.
Highlights
Early results across multiple dose-escalation and optimization cohorts highlight three consistent themes:
- Efficacy – Mipletamig has repeatedly produced more durable remissions in frontline AML, building on encouraging responses seen in earlier studies
- Safety – No dose-limiting toxicities or cytokine release syndrome have been observed to date, underscoring the differentiated safety profile enabled by Aptevo’s uniquely designed CRIS-7-derived CD3 binding domain
- Combinability – Mipletamig has demonstrated its ability to integrate with the venetoclax/azacitidine backbone, maintaining tolerability even at the highest dose levels studied
Outcomes to date reinforce mipletamig’s potential to become an important new component of frontline AML therapy and exemplify Aptevo’s mission to deliver safer, more effective immuno-oncology treatments to the market.
For the latest mipletamig news click here.
Why we are targeting CD123
Overexpression of CD123 is a mark of many forms of leukemia. Aptevo’s lead proprietary drug candidate, mipletamig is a bispecific CD3xCD123 ADAPTIR molecule that is designed to redirect the patients’ immune system to destroy leukemia cells expressing the surface target antigen CD123. This antibody-like recombinant protein therapeutic is designed to simultaneously engage both leukemia cells and T cells of the immune system to trigger the destruction of leukemia cells. Mipletamig has been designed with a unique CD3 binding domain to reduce the likelihood and severity of cytokine release syndrome (CRS). Mipletamig has received orphan drug designation for AML according to the Orphan Drug Act.
The Molecule
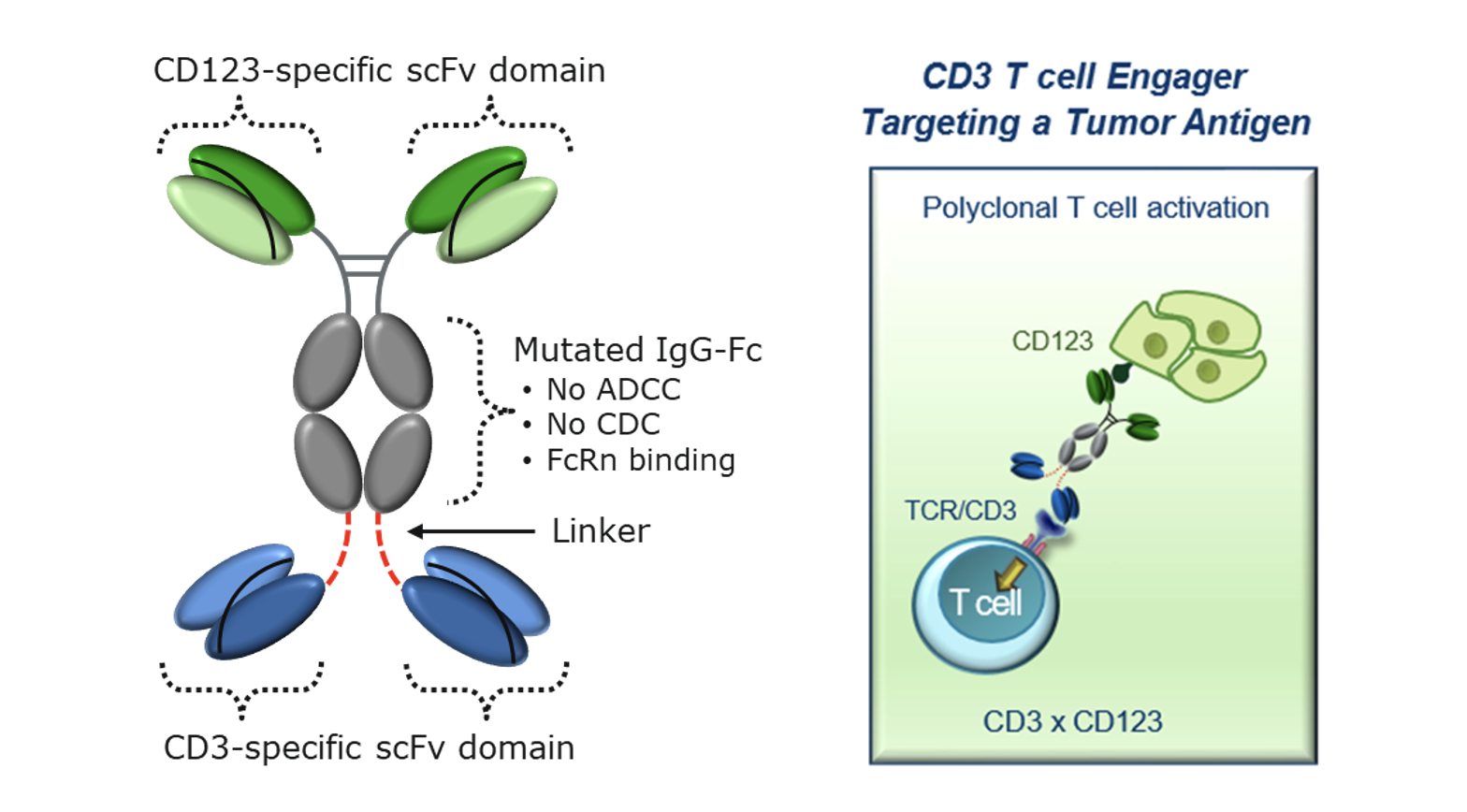
Potential advantages of Mipletamig include:
Broad Utility: The tumor-associated antigen CD123 is expressed on several hematological malignancies including acute myeloid leukemia, acute lymphoblastic leukemia, hairy cell leukemia, myelodysplastic syndrome, and blastic plasmacytoid dendritic cell neoplasm, which all have significant unmet needs for safe and effective new therapies.
Reduced Toxicity: The unique CD3 binding domain has high potency tumor killing while inducing a reduced cytokine release profile in vitro. This approach has been shown clinically to provide a safer and more tolerable treatment for AML patients.
Aptevo Expanded Access Policy (Oncology Programs)
Commitment to Patients
Aptevo is dedicated to developing innovative cancer therapies that have the potential to improve and extend the lives of patients with serious and life-threatening malignancies. Our mission is to bring safe and effective treatments to patients as quickly as possible through well-designed clinical trials and subsequent regulatory approval.
Policy Statement
At this time, Aptevo does not provide access to its investigational oncology therapies outside of clinical trials and therefore does not offer an Expanded Access (Compassionate Use) program.
Rationale
Our investigational oncology products are currently being evaluated in controlled clinical trials designed to understand their safety, efficacy, and appropriate dosing. Data from these trials are essential to obtain regulatory approval and to make the therapy broadly available to patients.
Providing investigational products outside of clinical trials at this stage could:
- Introduce safety risks that cannot be adequately monitored outside of a structured trial setting
- Compromise the integrity of clinical data, and
- Limit the availability of investigational drug supply needed to support ongoing studies
For these reasons, we believe that clinical trial participation is currently the most appropriate way for patients to access our investigational therapies.
Future Considerations
As development progresses and sufficient data becomes available, Aptevo will reevaluate this policy and reserves the right to revise this policy in whole or in part at any time, without notice. If the Company decides to initiate an Expanded Access Program in the future, details will be publicly posted on the Aptevo Website and ClinicalTrials.gov in accordance with the 21st Century Cures Act.
Clinical Trial Opportunities
Patients and physicians interested in our investigational oncology products are encouraged to review active clinical trials at:
https://clinicaltrials.gov/study/NCT06634394?term=APVO436&rank=3
Contact Information
For questions regarding our clinical programs or this policy, please contact:
Dirk Huebner, MD, Chief Medical Officer


